Advancing the Science of Cartilage Repair.
Zimmer Biomet’s OCA Kit contains fresh young allograft cartilage and cancellous chips. It is intended to provide surgeons with an early-intervention option to treat osteochondral defects and restore articular cartilage for multiple applications.
Procedures
- Cartilage Repair
Philosophies
- Early Intervention
Application
- Multiple
Zimmer Biomet fresh young allograft cartilage can significantly reduce pain and improve function for at least 5 years1,2
Survivorship at 5 years
85%in knees.1 |
Survivorship at 5 years
89%in knees.3 |
|---|
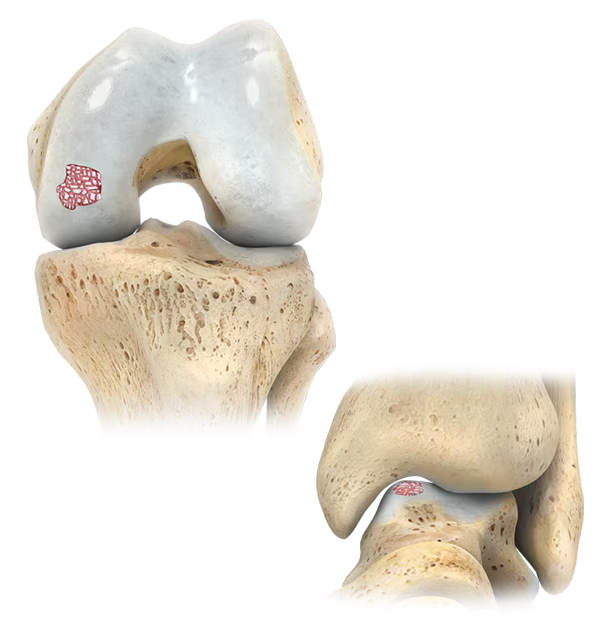
Product Features
The OCA Kit cartilage graft consists of young living articular cartilage, which contains 10x greater chondrocyte density than adult tissue and does not elicit an allogeneic immune response 4-6*
The modular graft makes it possible to address deficiencies of various sizes and shapes.
Specifications
For any inquiries regarding reimbursement, please contact Zimmer Biomet Reimbursement Hotline: Phone: 1-866-946-0444. Hotline Hours: Monday-Friday, 8am- 5pm, EST
Email: [email protected]
Benefits
Single-stage Procedure
- Allows for a single-stage procedure with fibrin fixation, eliminating the need for harvesting autograft or a periosteal flap – thereby reducing donor site morbidity and saving time in the OR
Fresh Young Allograft Cartilage Advantage
- Juvenile allograft cartilage contains 10x greater chondrocyte density than adult tissue and does not elicit an allogeneic immune response4-6*
High Five Year Survivership1,3
- Zimmer Biomet Longitudinal Data Collection (LDC) on fresh young allograft cartilage show survivorship at 5 years 85% in knee and 89% in ankle1,3

Additional Information
- Data on file Zimmer Biomet. Post-Market, Longitudinal Data Collection Study of DeNovo® NT for Articular Cartilage Defects of the Knee, protocol number CSU2010-22B.
- Farr, J., et al. Clinical, Radiographic and Histological Outcomes following Cartilage Repair with Particulated Juvenile Articular Cartilage: A 2-Year Prospective Study. American Journal of Sports Medicine. 42(6):1417-25, 2014.
- Data on file Zimmer Biomet. Post-Market, Longitudinal Data Collection Study of Articular Cartilage Lesions in the Ankle Treated with DeNovo NT Natural Tissue Graft, protocol number CSU2010-21B. Nov 13, 2017.
- Adkisson, H. D., et al. The potential of human allogenic juvenile chondrocytes for restoration of articular cartilage. American Journal of Sports Medicine. 38(7):1324-33, 2010.
- Federer, J., et al. The Promise of Chondral Repair Using Neocartilage. In Sandell, J. S., et al (red) Tissue Engineering in Musculoskeletal Clinical Practice. American Academy of Ortopaedic Surgeons. Chapter 22, pp. 219-226, 2004.
- Adkisson, H. D., et al. Immune evasion by neocartilage-derived chondrocytes: Implications for biologic repair of joint articular cartilage. Stem Cell Research. 4:57-68, 2010.
- Farr, J., et al. Chondral Defect Repair with Particulated Juvenile Cartilage Allograft. Cartilage. Oct 2(4):346-353, 2011.
- Coetzee, J. C., et al. Treatment of Osteochondral Lesions of the Talus With Particulated Juvenile Cartilage. Foot & Ankle International. Sep;34(9):1205-11, 2013.
*Cell assays are not necessarily indicative of clinical performance
Zimmer, Inc.
1800 West Center Street
Warsaw, Indiana 46580 USA