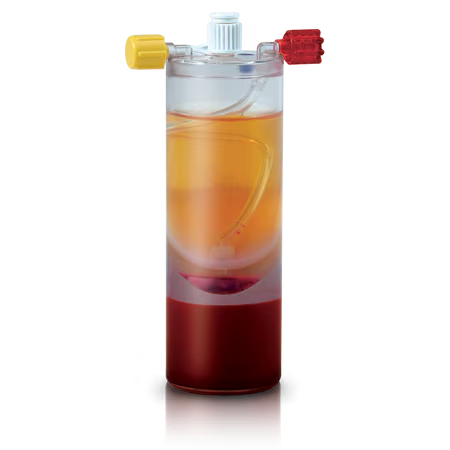
Unlike traditional PRP disposables which only process whole blood, the BioCUE System concentrates platelets and white blood cells from a combination of whole blood and bone marrow aspirate (bBMA).
Focus on Bone Marrow
Aspirated bone marrow is frequently used for bone grafting. Designed to process a mixture of autologous whole blood and bone marrow aspirate, the BioCUE bBMA Concentration System represents an evolution in this technique.
Procedures
- Autologous Therapies
Philosophies
- Early Intervention
Application
- Multiple
Average bBMA PRP Output* Concentrations
*The platelet-rich plasma (PRP) prepared by this device has not been evaluated for any clinical indications
Recovery
77.5%of nucleated cells1 |
Survivorship at 5 years
71%of available platelets1 |
|---|
Recovery
7.2xof available platelets1 |
Survivorship at 5 years
7.9xof available nucleated cells1 |
|---|

System Features
The BioCUE System includes all the components to draw blood, aspirate bone marrow, easily process the disposable system, and produce an autologous PRP output* to hydrate the surgeon’s choice of autograft and/or allograft bone.
* The PRP prepared by this device has not been evaluated for any clinical indications
Specifications
The bone marrow aspirate (BMA) needle provided with the BioCUE System has several advantages over traditional aspiration needles.
- 6 holes at the distal tip, allowing for more efficient aspiration
- Stylet with trocar point for penetration of the cortical bone into the bone marrow cavity
- Stylet with blunt tip for easy movement of the needle within the bone marrow cavity
Blood draw components are provided for the aspiration of whole blood using standard venipuncture techniques.
Benefits
All in One
- Includes all components necessary to obtain and process autologous bone marrow aspirate at a patient’s point-of-care
High Recovery Nucleated Cells
- Recovery of 77.5 percent of the nucleated cells from a bone marrow aspirate input1
Convenient Usage
- Dual buoy design eliminates need to filter bone marrow and whole blood prior to processing.
Additional Information
- BB2. Concentrations of Platelet-Rich Plasma and Bone Marrow Aspirate using the BioCue™ Platelet Concentration System and the Magellan™ Basic Platelet Separator Kit. 2010.
*The platelet-rich plasma (PRP) prepared by this device has not been evaluated for any clinical indications.
Biomet Orthopedics
56 East Bell Drive
P.O. Box 587
Warsaw, Indiana 46581 USA