In Harmony with Natural Anatomy
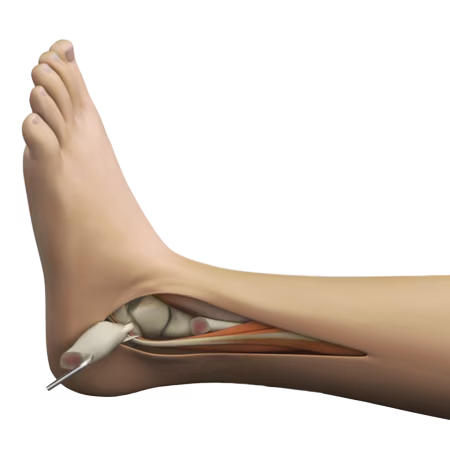
Created to meet surgeon and patient needs, this ankle implant features a low profile design, lateral surgical approach and industry-first Prolong® Highly Crosslinked Polyethylene.
Anatomy
- Ankle
Procedure
- Total Ankle Replacement
Advanced implant designed to last
- Prolong Highly Crosslinked Polyethylene bearing surface to reduce volumetric wear.3,4
- Trabecular Metal has a high coefficient of friction vs. cancellous bone for stable initial fixation.5
- Reduced bony resection with the TM Ankle leaves more bone, which Statistically significantly increases bony support when compared to the alternative, flat resection.2

System Features
Specifications
Sizing Options
6 Tibia and Talus Sizing Options
3 poly thickness options for each size
Benefits
Durability
- The TM Ankle demonstrates 97.3% implant survivorship at three year follow-up, favorable to the overall class of TAA.1,6
Additional Information
References
- Australian Orthopaedic Association National Joint Replacement Registry (AOANJRR). Demographics and Outcome of Ankle Arthroplasty: Supplementary Report in Hip, Knee, & Shoulder Arthroplasty: 2018 Annual Report, AOA, Adelaide; 2018: 1018. 2018 Annual Report. Adelaide, AOA, 2018. Table A8, Page 11. [Accessed from: https://aoanjrr.sahmri.com/annual-reports-2019/supplementary].
- Bischoff J, Fryman J, Parcell J, Orozco Villaeñpr D, Influence of Crosslinking on the Wear Performance of Polyethylene Within Total Ankle Arthroplasty. Foot & Ankle International 2014. Vol. 36(4), 369-376.
- Data on file at Zimmer.
- Gsell R, Yao JQ, Laurent MP, Crowninshield RD: Improved oxidation resistance of highly crosslinked UHMWPE for total knee arthroplasty. Society for Biomaterials 27th Annual Meeting Transactions, 84, 2001.
- Zhang Y, Ahn PB, Fitzpatrick DC, Heiner A, Poggie RA, Brown TD, Interfacial frictional behavior; cancellous bone, cortical bone, and a novel porous tantalum biomaterial. Journal Musculoskeletal Research 1999.
- Zimmer® Trabecular Metal™ Total Ankle Post-Market Clinical Follow-Up Study Annual Report 2019.