Two-Piece Design
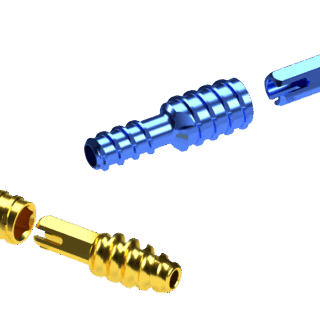
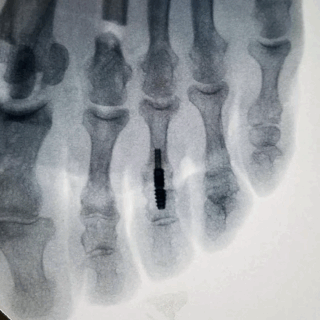
The two-piece cannulated design allows for optional technique to pin metatarsophalangeal joint
- Two-piece threaded implant construct designed for optimal bone purchase
- Cannulated implants and instruments provide targeting and technique guidance for repeatable procedures
- Implant-to-implant rotational stability via differentiated hexagonal locking design
- Variable implant locking position provides in-situ adjustability before final closure
- Allows for optional technique to pin metatarsophalangeal joint
- Single-use sterile packed kit
Specifications
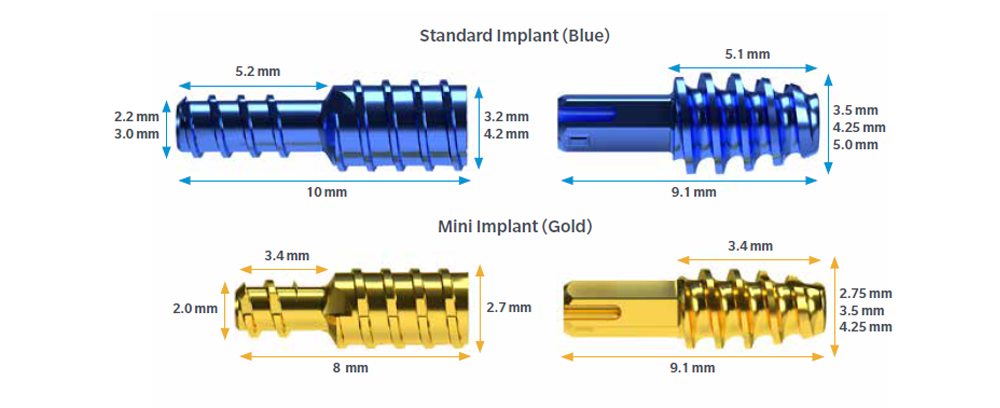
Standard
- 3.2mm and 4.2mm proximal diameter
- 3.5mm, 4.25mm, 5.0mm diameters in middle phalynx
Mini
- 2.7mm proximal diameter
- 2.75mm, 3.5mm, and 4.25mm diameters in middle phalynx
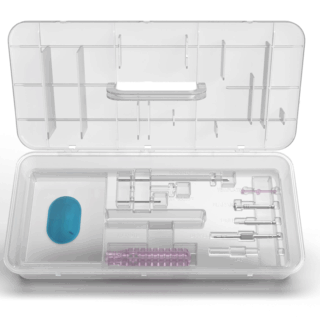
- Single-use instrument tray
- Designed for precise, reproducible procedures
- Optimized for OR efficiency
- Sizing guide for canal diameter
Additional Information
Distributor and Manufacturer
Paragon Medical Inc.
8 Matchett Drive
Pierceton, IN 46562
Tel: (800)255-6975



