Complements the body’s natural healing processes and encourages normal bone structure
Unrivaled flexibility for delivery and application
Completely absorbs to leave no trace
In 12 months, genex is completely absorbed and remodeled while leaving no foreign artifacts after resorption1,2
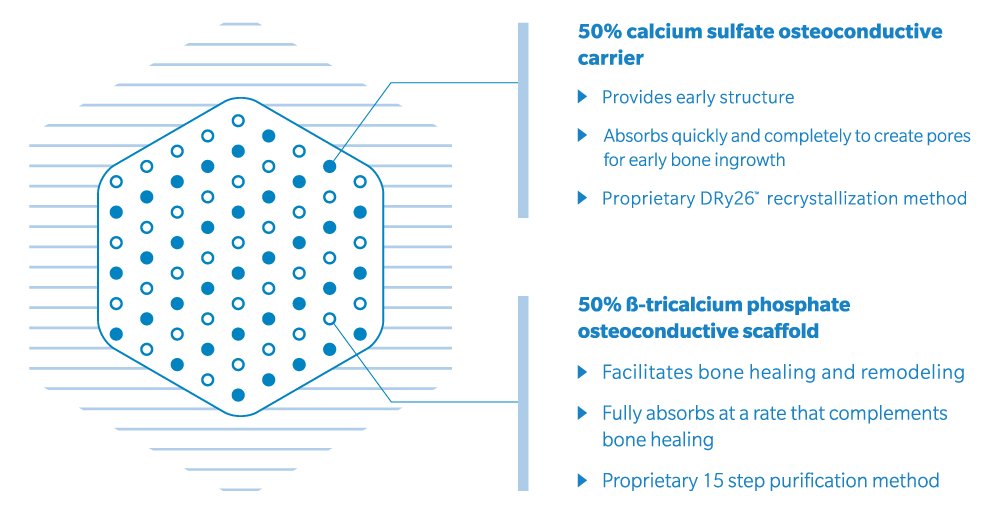
Biphasic composite of purity, balance, and characteristics
Our proprietary recrystallization and purification methods removes impurities from genex such as6
- No inflammatory pyrophosphates
- No slow and non-absorbing compounds such as hydroxyapatite
Several studies have concluded healthy bone is restored in a clinically relevant timeframe7-9
genex is a precisely balanced ß tricalcium phosphate/calcium sulfate hemihydrate compound with distinct design properties:
- Contains no hydroxyapatite (HA)
- Negatively charged surface chemistry
- Compressive strength similar to trabecular bone
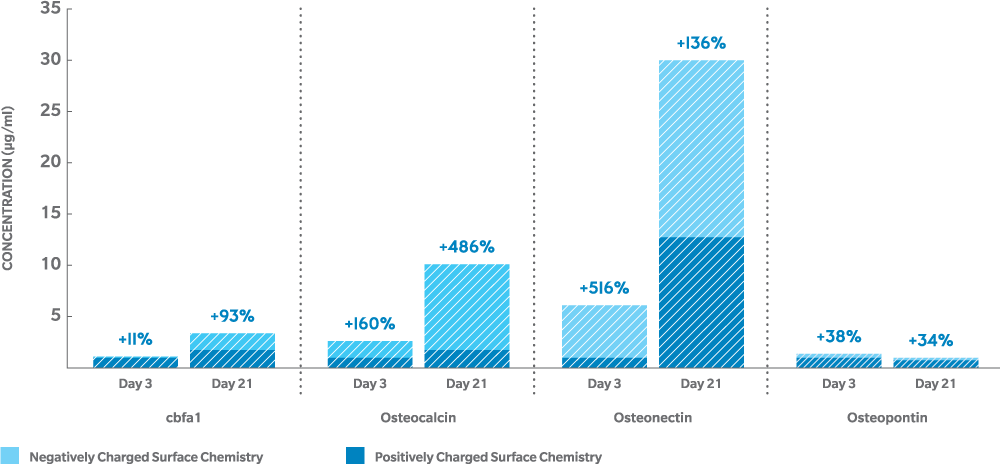
Negatively charged genex enhances the osteogenic response to accelerate bone growth10,14
- genex containts no hydroxyapatite.
- HA can only be absorbed at 1-2% per year12
- up to 5 times normal levels (on the image):
- In-vitro human osteoblast culture, 3 days11
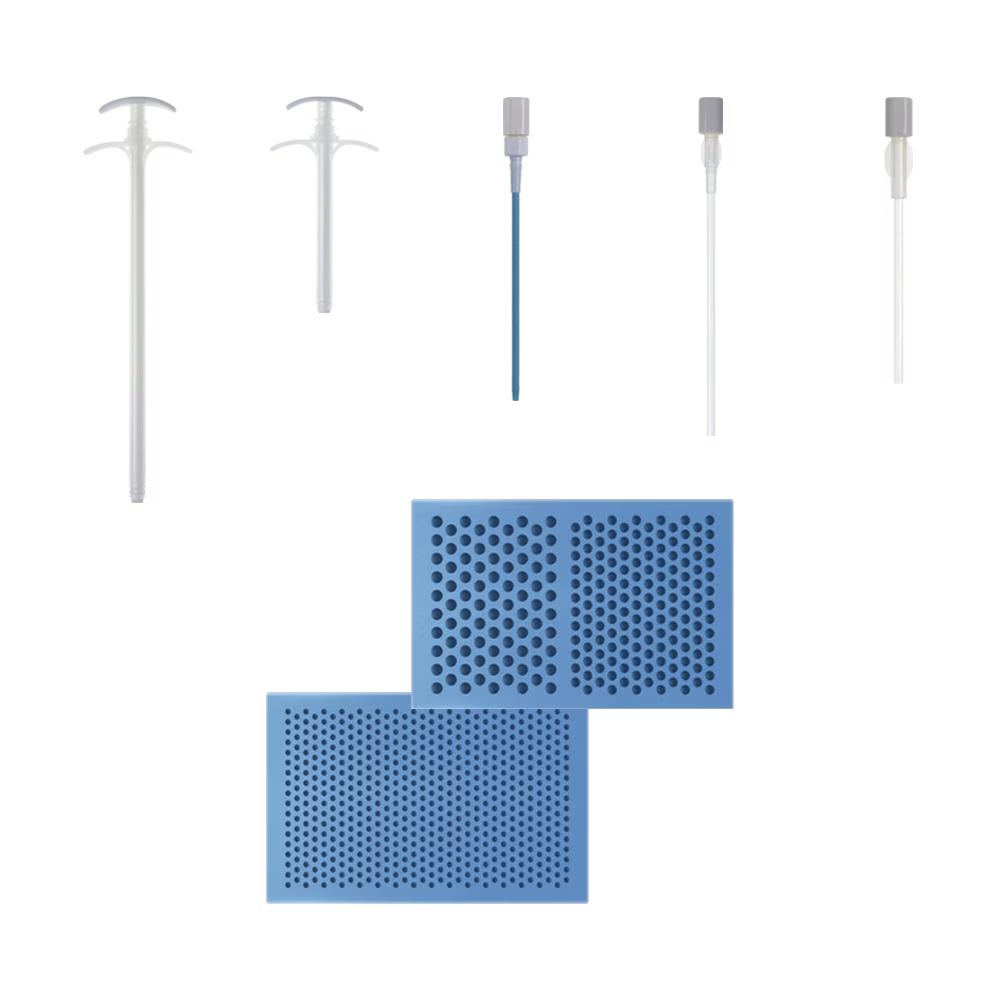
System Features
Comprehensive Autologous Bone Graft Delivery
- Unrivaled flexibility of delivery and application
- Closed-mixing system is 2x faster to prepare than open mixing system13
- Longer working time
- Drillable 15 minutes after mixing
Specifications
3 Sizes
- 3cc
- 5cc Kit
- 10cc Kit
Mold Beads
- Can be made in 3mm, 4.8mm, or 6mm diameters
- 5cc Pack = 10cc Bead volume
- 10cc Pack = 20cc Bead volume

Cannula Options
- 6mm ID, 8mm OD, 157mm IL
- 6mm ID, 8mm OD, 72mm IL
- 2.1mm ID, 3.omm OD, 79mm IL
- Radiopaque, Tapered (12G)
- 2.5mm ID, 3.5mm OD, 100mm IL (11G)
- 3.15mm ID, 3.75mm OD, 70mm IL (9G)
Additional Information
References
- Yang HL et al. Bone healing response to a synthetic calcium sulfate/ß-tricalcium phosphate graft material in a sheep vertebral body defect model. J Biomed Mater Res B Appl Biomater 2012;100B(7):1911–21.
- Clinical case study: Mr A Nisar and Mr S Gopal; Proximal femur fracture, Data on file.
- Biocomposites internal testing: Biomaterials Compressive strength; Applicable methodology ISO/DIS 18531:2015(E) Implant for surgery – Calcium phosphate bioceramics – Characterization of hardening bone paste materials. 2015, MA0390R1.
- Misch CE, Qu Z, Bidez MW. Mechanical properties of trabecular bone in the human mandible: implications for dental implant treatment planning and surgical placement. J Oral Maxillofac Surg. 1999 Jun;57(6):700 6; discussion 706-8. doi: 10.1016/s02782391(99)90437-8. PMID: 10368096
- Dunham CE, Takaki SE, Johnson JA, Dunning CE. Mechanical properties of cancellous bone of the distal humerus. Clin Biomech (Bristol, Avon). 2005 Oct;20(8):834-8. doi: 10.1016/j. clinbiomech.2005.05.014. PMID: 16023773.
- Biocomposites internal and external testing: genex Bone Graft Substitute technical file: Section 3.1.7 (Rev1) Final product specification, 2022.
- Clinical case study: Mr HK Sharma; Tibial plateau fracture: Data on file.
- Clinical case study: Prof JB Richardson; Distal tibia non-union: Data on file.
- Clinical case study: Mr P Thompson; Single stage revision ACL reconstruction: Data on file.
- Cooper JJ et al. Enhancing the osteogenic potential of bioabsorbable implants through control of surface charge. Presented at the Society for Biomaterials 2007 Annual Meeting, April, 2007: Chicago, Illinois, USA.
- J J Cooper, J A Hunt., The Significance of Zeta Potential in Osteogenesis. Poster presented at Society for Biomaterials 2006 Annual Meeting, Pennsylvania, USA.
- Pina S, Ferreira JMF. Bioresorbable plates and screws for clinical applications: A review. J Healthcare Engineering 2012;3(2):243–60.
- Biocomposites internal and external testing: genex Bone Graft Substitute technical file: section 4.5.1.28 genex Bone Graft Substitute usability time evaluation study, 2022.
- Stein JM, Fickl S, Yekta SS, et al. Clinical Evaluation of a Biphasic Calcium Composite Grafting Material in the Treatment of Human Periodontal Intrabony Defects: A 12-Month Randomized Controlled Clinical Trial. J Periodontol. 2009 Nov; 80 (11): 1774-82. doi: 10.1902/jop.2009.090229.
Dry26™ genex™ are trademarks of Biocomposites, LTD



