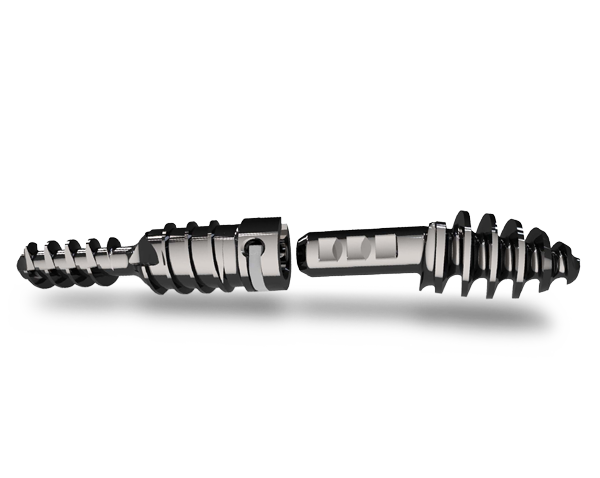
Two-piece Design
The two-piece design with RevLock® Adjustable Locking Mechanism provides intermediate locking before final closure and allows for retained compression.
Maximized bone purchase1
- Stable and secure phalanx
- Designed to optimize screw design
- Adjustable bone to bone apposition
- Progressive ratchet tightening mechanism
- Intended to aid bone fusion
2-piece precision insertion
- Intermediate locking before final closure
Reversible assembly
- Proprietary RevLock Adjustable Locking Mechanism
- Natural anatomically correct design with 10 degree angulation
Superior Bone Purchase vs. K-wires2
Designed to increase fusion rate, activity, quality of life and reduce pain.3
System Features
Specifications
3 Sizes for Density and Width
3.5mm
Nextra® Hammertoe Sterile Implant: 3.5mm Middle Phalanx Implant/ 3.2mm Proximal Implant
4.5mm
Nextra® Hammertoe Sterile Implant: 4.5mm Middle Phalanx Implant/ 3.2mm Proximal Implant
5.5mm
Nextra® Sterile Middle Implant: 5.5mm Middle Phalanx Implant
Benefits
Stability
- Designed to maximum bone purchase1, stabilize and secure phalanx, and optimize screw design for repeatable outcomes.2
Versatile
- This system is able to be adjusted in situ and is removable at surgery offering the ability to revise if position is not optimal. The adjustable bone-to-bone apposition with progressive ratchet tightening mechanism and reversible assembly are delivered using RevLock®.
Anatomic
- Anatomically correct design with 10 degrees of angulation to restore to natural state.
Additional Information
References
- “Achieving Maximum Phalangeal Fixation and Bone Contact through Osteological Design of Hammertoe Implants”; Osteological study of bone purchase for the Nextra Hammertoe Implant.
- Jay RM, Malay DS, Landsman AS, Jennato N, Huish J, Younger M. “Dual-Component Intramedullary Implant versus Kirschner Wire for Proximal Interphalangeal Joint Fusion: A Randomized Controlled Clinical Trial”- The Journal of Foot & Ankle Surgery 55 (2016); 697-708.
- Jay, Richard, et al. Prospective Radomized Multicenter Comparative Effiveness Study; Two Component Modular Digital Implant Versus K-Wire Fixation For Surgical Correction of Hammertoes.Viewed 2021.
Nextra® and RevLock® are trademarks of Nextremity Solutions, Inc.
Legal Manufacturer
NextremitySolutions
210 N. Buffalo St.
Warsaw, IN 46580 U.S.A.