Offering an Early Treatment Option for Your Patients
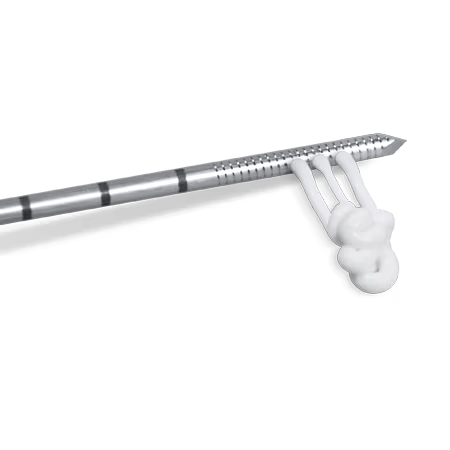
The Subchondroplasty Procedure (SCP) is a minimally-invasive, fluoroscopically-assisted intervention that targets and fills subchondral bone defects, often called Bone Marrow Lesions (BML) with AccuFill® Bone Substitute Material.
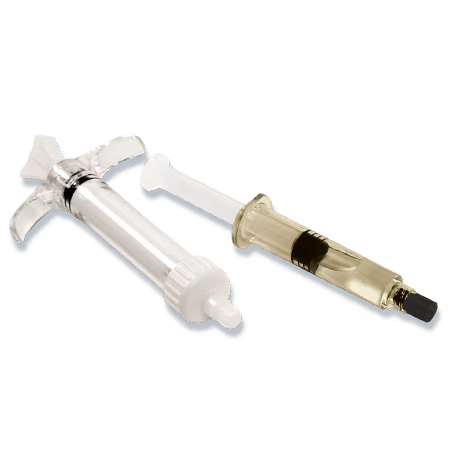
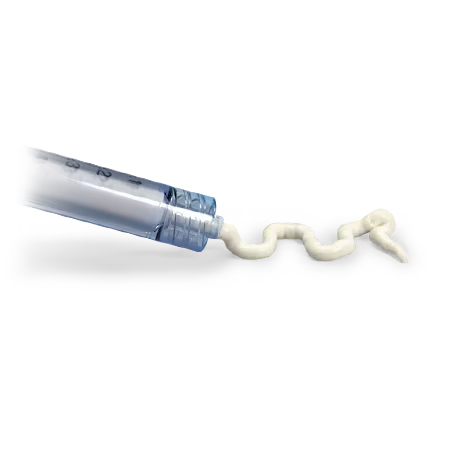
AccuFill BSM is an engineered calcium phosphate mineral compound, a bone graft substitute, that mimics the properties of cancellous bone, resorbs and is replaced with new bone during the healing process.1,2
The SCP Procedure is usually performed along with arthroscopy for visualization and treatment of findings inside the joint. In some cases, an open or mini-open procedure is necessary for access to the defect.
Philosophies
- Early Intervention
Application
- Knee, Foot & Ankle, Hip, Shoulder
Peer-reviewed data demonstrate sustained pain reduction, improved function and quality of life after the SCP Procedure.3,4
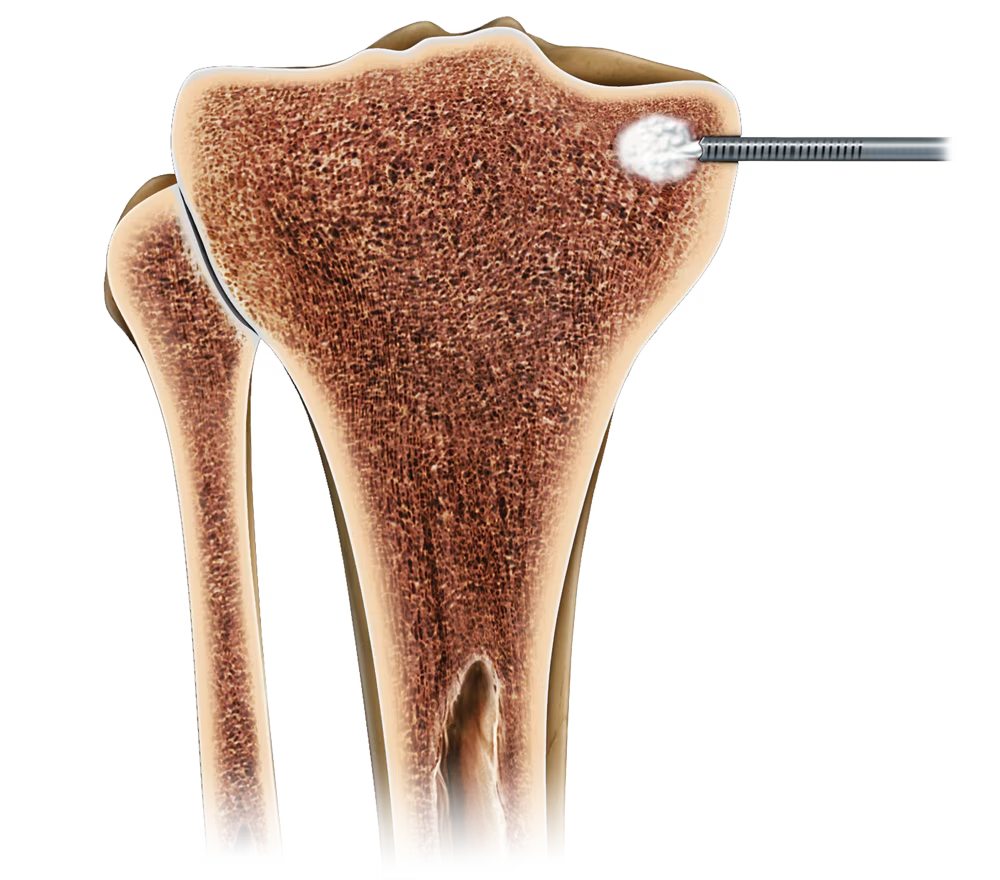
System Features
The minimally-invasive SCP procedure is usually performed along with arthroscopy for visualization and treatment of findings inside the joint. In some cases, an open or mini-open procedure is necessary for access to the defect.
Bone defects are filled with AccuFill Bone Substitute Material (BSM), a proprietary, flowable, biomimetic bone substitute.1,2
AccuFill BSM is available in prefilled (PF) mixing and saline syringes. The prefilled kit includes all components to hydrate and mix AccuFill BSM in a closed mixing system.
*The grain size of the hydroxyapatite (HA) crystals that form as part of the amorphous and crystalline mixture of calcium phosphate sets are on the nanometer scale. The size of the crystalline structures were measured by X-ray diffraction to be less than 100 nanometers.
Specifications
AccuFill BSM is an engineered calcium phosphate mineral compound.1 It flows readily to fill subchondral defects, crystallizes and hardens quickly in an Isothermic reaction at 37°C to form a nanocrystalline, macroporous scaffold in the bone.
Over time, through cell-mediated remodeling, AccuFill BSM is resorbed and replaced with new bone.2
Important Information: The use of AccuFill BSM is not intended to be intrinsic to the stability of the bony structure. Radiographic imaging should be used to confirm that the adjacent cortical bone is intact.
AccuFill Injectable Bone Substitute Material is an injectable, self-setting, macroporous, osteoconductive, calcium phosphate bone graft substitute material that is intended for use to fill bony voids or gaps of the skeletal system of the extremities, spine (i.e. posterolateral spine), and pelvis that are not intrinsic to the stability of the bony structure. These defects may be surgically created osseous defects or osseous defects created from traumatic injury to the bone. AccuFill Injectable Bone Substitute Material is a bone graft substitute that resorbs and is replaced with new bone during the healing process.*
Patients typically present with:
- Presence of bone defect seen on fat-suppressed MRI (T2FS, PDFS, STIR, etc.)
- No resolution of BML with conservative care or other intervention
Discuss all treatment options with your patient. All surgical procedures carry a certain level of risk. A complete assessment of possible risks should be discussed before making the decision to have surgery.
Benefits
Application
- Treats chronic, symptomatic, subchondral bone defects which are the primary cause of joint pain, not the intra-articular tissue.5,6
Treatment
- Proprietary minimally-invasive, joint preserving injection of AccuFill BSM which is replaced with new bone during the healing process.1,2
- Usually performed along with arthroscopy to aid in visualization, the SCP procedure does not inhibit the treatment of other findings inside the joint when performed along with arthroscopy.
- Typically, an outpatient procedure enabling patients to generally return home the same day.
Results
- AccuFill BSM sets hard and remodels into new bone during the healing process.1,2
- Peer-reviewed data demonstrate sustained pain reduction, improved function and quality of life.3,4
- SCP Procedure does not preclude future procedures. Should it become necessary, future surgery is still an option.
Additional Information
- Tofighi A., et al. New Generation of Synthetic, Bioresorbable and Injectable Calcium Phosphate Bone Substitute Materials: Alpha-bsm, Beta-bsm and Gamma- bsm. Journal of Biomimetics, Biomaterials and Biomedical Engineering (JBBBE). 2:39, 2009.
- Angle SR, et al. Novel Macroporous Calcium Phosphate Scaffold To Improve Cell Infiltration and Osseous Integration. Transactions of the 61st Annual Meeting of the Orthopaedic Research Society: 1157, 2015.*
- Cohen SB, et al. Subchondroplasty for Treating Bone Marrow Lesions. Journal of Knee Surgery. Oct;29(7):555-563, 2016.
- Astur DC, et al. Evaluation and Management of Subchondral Calcium Phosphate Injection Technique to Treat Bone Marrow Lesion. Cartilage. Oct;10(4):395-401, 2019.
- Hunter DJ, et al. Evolution of semi-quantitative whole joint assessment of knee OA: MOAKS (MRI Osteoarthritis Knee Score). OA & Cartilage. Aug; 19(8) 990-1002, 2011.
- Sowers M, et al. Associations of Anatomical Measures from MRI with Radiographically Defined Knee Osteoarthritis Score, Pain, and Physical Functioning. Journal of Bone and Joint Surgery Am. 93:241-251, 2011.
AccuFill BSM
ETEX Corporation
55 Messina Drive
Braintree, MA 02184
USA