Large volume
Rapid delivery
Minimally invasive technique
DragonWing™ is a sterile-packed, single-use delivery kit enabling percutaneous bone graft delivery. The DragonWing enables surgeons to streamline bone graft delivery minimally invasively with reloadable graft cartridges.
Indications for Use:
- To be used for the delivery of autograft or hydrated allograft bone graft material to an orthopaedic surgical site.
Procedure Type Examples:
- Fusions
- Nonunions
- Arthroscopic/MIS Grafting
- Cysts
- Bone Voids
- AVN
- Lesions
System Features
Comprehensive Autologous Bone Graft Delivery
- Straight delivery cannulas in kit for percutaneous autograft delivery
- Multiple graft cartridges in kit to for ease of insertion
- Multi channel cartridge design for streamlined delivery process

Specifications
Disposable Instrument Kit
| Item | Description | Part No. |
| 1 | Avitus DragonWing Large Volume Autograft Delivery | AD-5ch-5.2-120 |
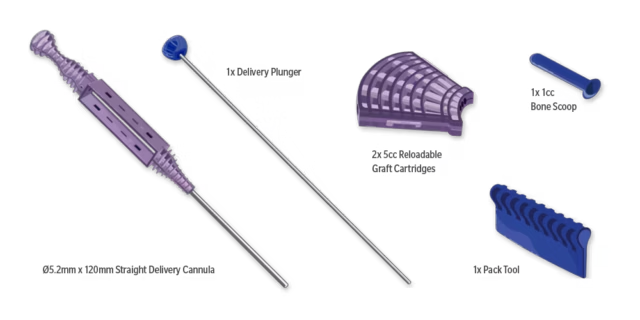
What's in the Box
| Qty | Description |
| 2 | Graft Cartridge (5cc each) |
| 1 | Straight Delivery Cannula (5.2mm x 120mm) |
| 1 | Delivery Plunger |
| 1 | Pack Tool |
| 1 | Bone Scoop (1 x 1cc) |
Benefits
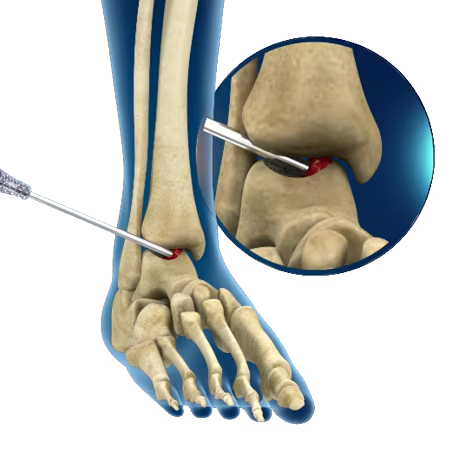
Minimally Invasive
The system offers a straight delivery cannula to percutaneously delivery autologous bone.
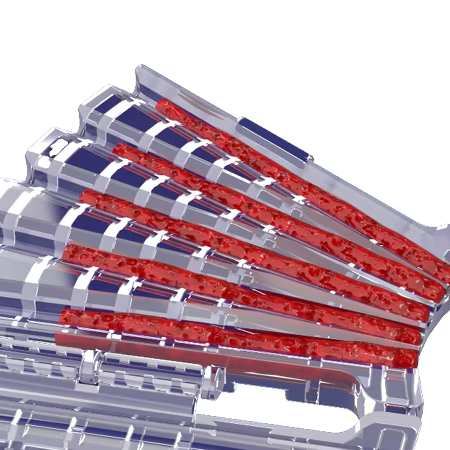
Large Volume
The large volume multi-channel graft cartridges allow for 5cc’s of bone graft to be preloaded for easy insertion.

Rapid Delivery
The multiple graft cartridges allow smooth, controlled and quick delivery of autograft.
Additional Information
References
Refer to IFU for complete list of indications and contraindications.
Distributor and Manufacturer
Distributor
Zimmer, Inc.
1800 West Center St.
Warsaw, IN 46580 U.S.A
Manufacturer
Avitus Orthopaedics Inc.
6 Armstrong Rd. 2nd Floor
Shelton, CT 06484





