Precise volume
Minimally invasive
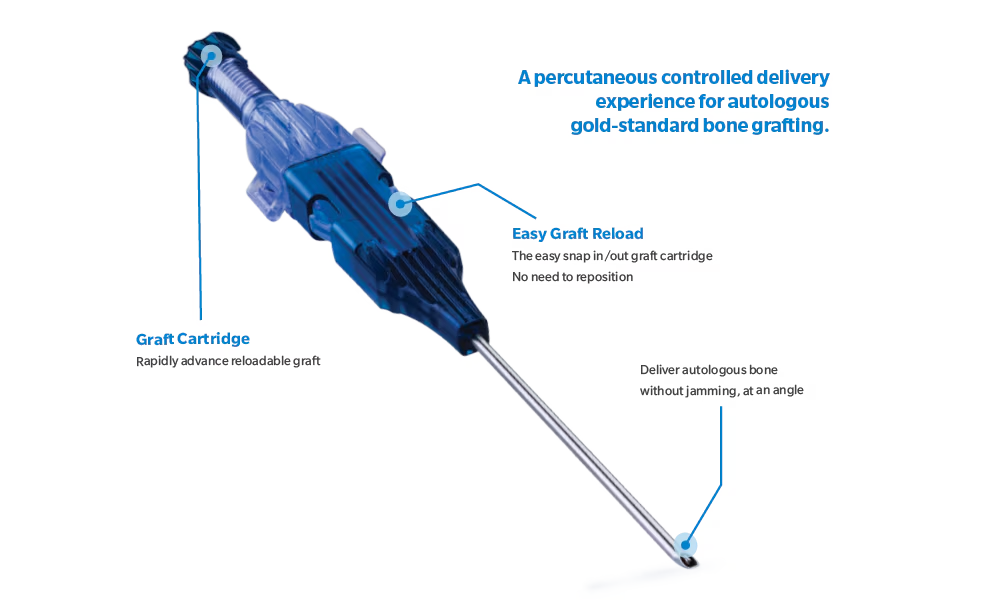
Controlled delivery
ArchiMIS™ is sterile-packed, single-use delivery kit enabling percutaneous bone graft delivery. Surgeons are able to precisely control small volume bone graft deployment.
Indications for Use:
- To be used for the delivery of autograft or hydrated allograft bone graft material to an orthopaedic surgical site.
Examples:
- OCD bone grafting
- Arthroscopic autograft delivery
- MIS/Percutaneous autograft delivery
- Bone marrow lesions
- MIS/Percutaneous fusions
- Bone lesions and cysts
- Osteochondral lesions
- MIS fusions
System Features
Comprehensive Autologous Bone Graft Delivery
- Straight and curved delivery cannulas available in kit for percutaneous autograft delivery
- Multiple graft cartridges to allow for streamlined delivery process
- Threaded delivery plunger for precise and controlled delivery of bone graft
Specifications
Disposable Instrument Kit
| Item | Description | Part No. |
| 1 | Avitus ArchiMIS Precision Autograft Delivery | AD-1ch-4.0-120 |
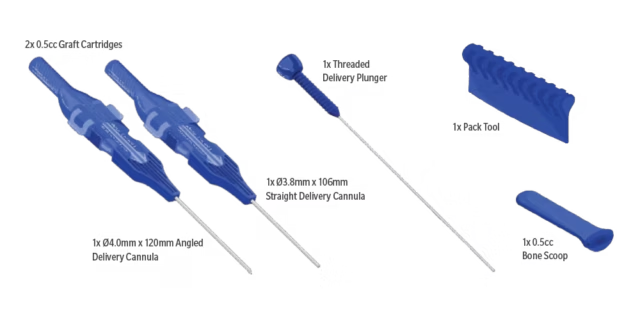
What's in the Box
(AD-1ch-4.0-120)
| Qty | Description |
| 2 | Graft Cartridge (0.5cc each) |
| 1 | Straight Delivery Cannula (3.8mm x 106mm) |
| 1 | Angled Delivery Cannula (4.0mm x 120mm) |
| 1 | Threaded Delivery Plunger |
| 1 | Pack Tool |
| 1 | Bone Scoop (1 x 0.5cc) |
Benefits
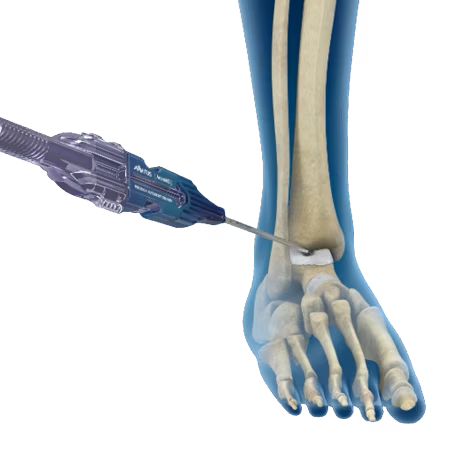
Minimally Invasive
The system offers both a straight and angled delivery cannula to percutaneously delivery autologous bone at various insertion angles.
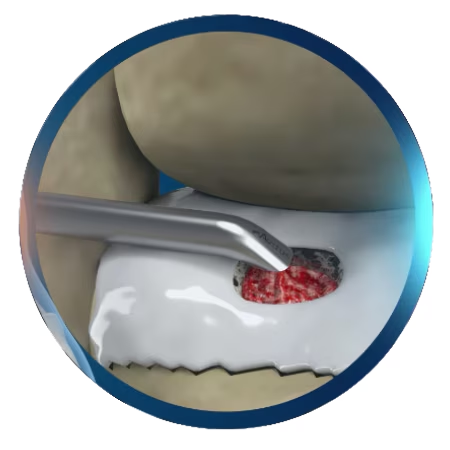
Precise Volume
The graft cartridges allow for precise volumes of 0.5cc’s each to be preloaded for easy delivery.

Controlled Delivery
The threaded delivery mechanism offers precise control of bone graft deployment.
Additional Information
Distributor and Manufacturer
Distributor
Zimmer, Inc.
1800 West Center St.
Warsaw, IN 46580 U.S.A
Manufacturer
Avitus Orthopaedics Inc.
6 Armstrong Rd. 2nd Floor
Shelton, CT 06484





