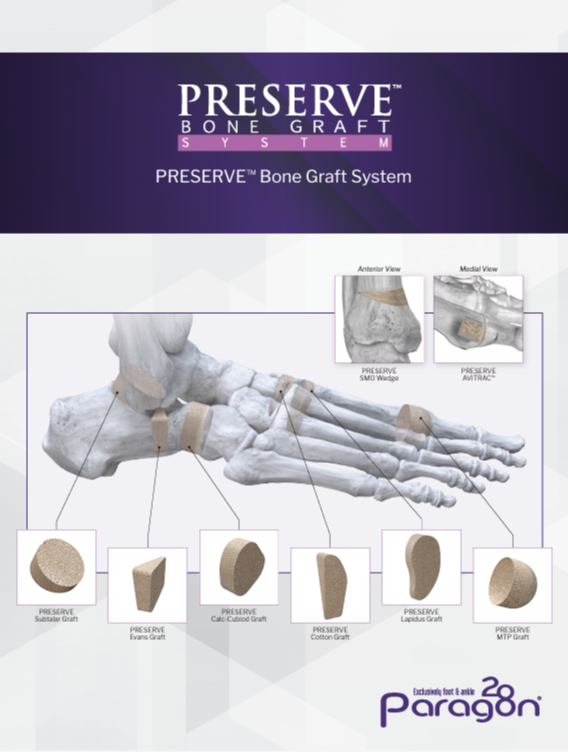
BiologicsPRESERVE™ Evans Lateral Column Lengthening Graft
PRESERVE™ Evans Lateral Column Lengthening Graft
PRESERVE™ Evans Lateral Column Lengthening Graft
PRODUCT DESCRIPTION
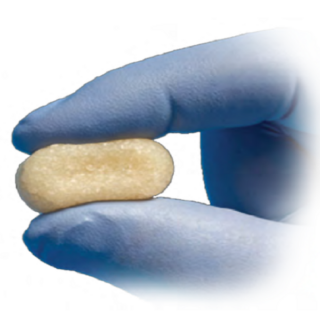
The PRESERVE™ Evans Lateral Column Lengthening Graft is a patented, tapered wedge designed for use in an Evans Calcaneal Osteotomy. The Paragon 28® PRESERVE™ Grafts are anatomically contoured, aseptically processed and density matched allografts. The anatomic contour of the Evans graft features a dorsal to plantar taper as well as a more standard lateral to medial taper. This allows for ease of insertion and offloading of surrounding soft tissue structures. The primary donor sites for the PRESERVE™ Evans Lateral Column Lengthening Graft are the patella, talus and femoral calcar to provide strength to an area subject to high compressive loads.
All Paragon 28® PRESERVE™ Grafts are aseptically processed, allowing for maintenance of structural integrity and biocompatibility of the graft. Gamma irradiation is not used during processing in order to help avoid structural fatigue and crumbling of the graft. Further, hydrogen peroxide is avoided during processing with the intent to help preserve osteoinductivity of the graft.
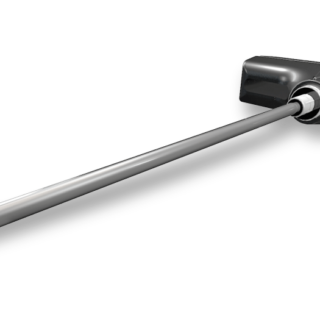
A set of trials are available for use with the PRESERVE™ Evans Lateral Column Lengthening Grafts. The trials are sized matched to the grafts to determine the correct amount of correction both clinically and radiographically before a graft is even opened.
Paragon 28®’s Design Rationale
We listened to surgeons when they worried about patients experiencing lateral column pain following an Evans Calcaneal Osteotomy. Through discussions with surgeons and anatomic research, a dorsal to plantar taper was included on the PRESERVE™ Evans Lateral Column Lengthening Graft to help prevent over-stretching of the long plantar ligament and jamming of the calcaneocuboid joint, with the intention to reduce the incidence of post-operative lateral column pain.