Nail SystemsPhantom® Intramedullary Nail
Phantom® Intramedullary Nail
Phantom® Intramedullary Nail
PRODUCT DESCRIPTION
The Phantom® Intramedullary Nail is the first Paragon 28® intramedullary nail and offers a unique option for correcting hallux valgus at the first TMT joint. The nail and threaded peg construct is zero profile and minimizes the soft tissue disruption of the first metatarsal and medial cuneiform during a Lapidus procedure.
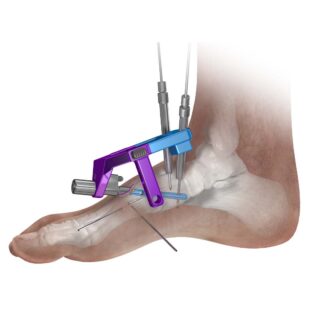
The Phantom® Intramedullary Nail instrumentation system provides unique targeting guides for precise placement and position of the nail and pegs. The polyaxial “claw shaped” guide facilitates the proper entry and angulation for drilling through the first metatarsal, TMT joint and into the medial cuneiform for proper nail placement and length. The outrigger and the outrigger slide allow targeting, drilling and entry of the pegs through the nail.
Paragon 28®’s Design Rationale
The Phantom® Intramedullary Nail was developed to provide surgeons with an intramedullary option for bunion surgery and correcting hallux valgus at the first TMT joint. The nail and threaded peg construct offer crossed screw fixation proximally and distally, generating a higher compressive force across the fusion site compared to traditional plate and screws¹. The zero profile design reduces soft tissue disruption, damage to the periosteum and reduces plantar gapping.
The nail is 5.5 mm in diameter, available in 12 options ranging from 38 mm to 60 mm in 2 mm increments and available in right and left specific configurations. The system offers 3.5 mm threaded pegs and 3.5 mm threaded locking pegs.
References
1 Test Report (TR-17060501) on file at Paragon 28®
Patient Story Video
Opting for bunion surgery with the innovative Phantom® Small Bone Intramedullary Nail was a pivotal decision for Ginger. This cutting-edge solution offered a minimally invasive approach, which may lead to quicker recovery and less post-operative discomfort. Ginger’s experience highlights the life-changing potential of advanced medical technology.
Post-surgery, Ginger experienced a profound improvement in her quality of life. With her bunion corrected by the Phantom® Nail she experienced restored confidence and was able to embrace an active lifestyle once again. Ginger’s story is a testament to the power of perseverance and the transformative impact of innovative medical solutions.
Surgical Video
Whether you are new to the system or looking to refine your technique, this video serves as a valuable resource to maximize surgical efficiency and patient outcomes.