Syndesmosis / Soft TissueTenoTac® 2.0 Soft Tissue Fixation System
TenoTac® 2.0 Soft Tissue Fixation System
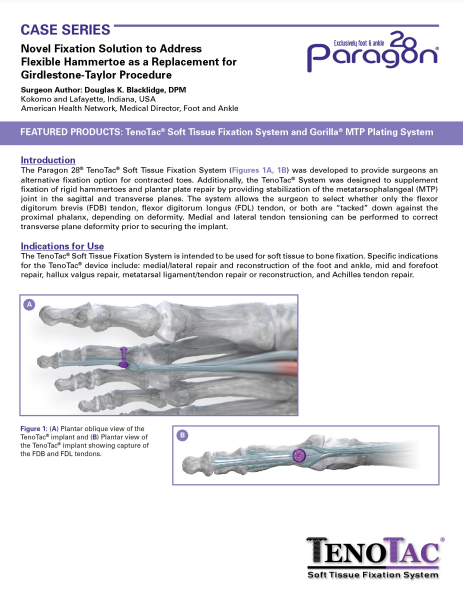
TenoTac® Soft Tissue Fixation System
TenoTac® 2.0 Enhancements:
Paragon 28 launched the TenoTac® 2.0 system to provide for greater capture of soft tissue, streamlined tensioning , and improved fit of the implant to the bony surface.
-
-
Plantar component includes a washer to allow it to angulate up to 20 degrees to contour to the plantar phalanx to maximize the amount of surface area the implant has on the flexor tendons and bone
-
Plantar component diameter increased to ensure increased capture of all four bands of the flexor tendons
-
Plantar component tooth height increased to allow for better capture and increased hold of the flexor tendons
-
Plantar inserter includes a sleeve to flush the drill tunnel of debris
-
Dorsal sleeve and plantar tack are tapered to allow for an easier mating of the components
-
Dorsal component is rectangular in shape with an increased surface area to limit plunging
-
Dorsal component has teeth to allow for capture of the dorsal cortex to limit plunging and to allow for the dorsal component to bite into the cortex while tightening to limit prominence
-


PRODUCT DESCRIPTION
TenoTac® is a patent-pending procedure and soft tissue fixation system designed to permanently address the source of the toe contracture. Cannulated instrumentation allows for accurate and reproducible placement of the implant. A threaded titanium implant with a spiked base is used to achieve and hold soft tissue fixation. The procedure requires minimal steps to correct flexible and semi-rigid hammertoe, plantar plate insufficiency, MPJ instability, as well as other applications in the forefoot. Instrumentation facilitates a two-handed technique to achieve correction, temporary fixation, and final implant placement. The implant features a low-profile dorsal component to reduce soft tissue irritation which threads into a plantar tack with a single row of spikes intended to grip and hold tension on the flexor tendon. The system is available in sterile kit offers two dorsal lengths to accommodate varying patient anatomies.