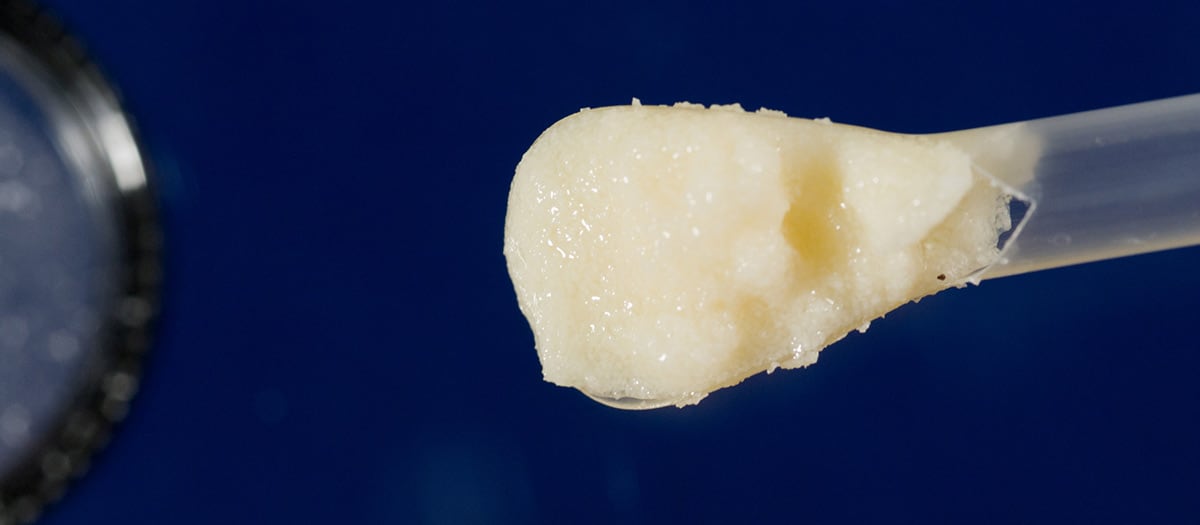
Paragon 28® is pleased to announce the launch of the V92 Cellular Bone Matrix. V92 is a viable allogeneic bone scaffold derived from bone marrow, in full compliance with FDA guidelines regarding human cells, tissues and cellular tissue-based products, and is intended for homologous use as a bone void filler.
Key Features
- Two unique scaffold blends for optimal handling characteristics: V92™ and V92™-FC
- Proprietary, optimized bone microparticulate size range of 100-300 μm.2
- Novel DMSO-free cryoprotectant, with no rinsing and decanting steps required prior to use.
- Average cell viability of the cell component exceeds 80% post-thaw.3
- Minimum of 150,000 viable cells per cc of allograft post-thaw.3
- Convenient handling and preparation in the OR, with total preparation time on the back table less than 20 minutes.
- Four (4) hour working window for implantation after thaw without loss of cell viability.
- Product shelf-life is two (2) years from date of processing when stored at -65°C or colder.
- Gruskin, E. et.al., Demineralized bone matrix in bone repair: history and use. Advanced Drug Delivery Reviews, 2012. 64:1063-1077.
- Malinin, T.I., et. al., Particulate bone allograft incorporation in regeneration of osseous defects; importance of particle sizes. The Open Orthopeadics Journal, 2007. 1:19-24.
- Data on file at Vivex Biomedical, Inc.
*Data on File – UMTB Biomedical